orange book pharmacy definition
2 approved over-the-counter OTC drug products for those drugs that. It is prepared by The Orange Book Staff.

Diabetes Without Drugs The 5 Step Program To Control Blood Sugar Naturally And Prevent Diabetes Complications Cohen Suzy 9781605296753 Amazon Com Books
The orange book is published annually and the 2015 edition is 35th edition of.
. Objectives What is a. Office of Generic Drugs Policy Center for Drug Evaluation Research US. Rucha Pathak Roll No.
Page 1 Definition It is the publication of Approved Drug Products with. Sumanta Mondal_MPharm 1 Sem. If youre a pharmacy student or working in the field youve probably heard of the Orange Book.
Multicultural Pharmacy Scholars Program. The orange book is a list of generic drugs approved by FDA. Search the Orange Book Database.
An introduction a how to use section the drug product lists appendices and a patent and exclusivity information addendum. Formally known as Approved Drug Products with Therapeutic. The electronic availability of the Orange Book brings this valuable tool to the web for.
For more information on the Orange Book including its history see the Orange Book Preface. Sumanta Mondal_MPhar m 1 th Sem. New Delhi India Dec 6 ANI.
_ GITAM Institute of Pharmacy. Food and Drug Administration. In the electronic Orange Book a reference standard is identified by RS in the RS column.
Search approved drug products by active ingredient. _ GITAM Institute of Pharmacy. But what is it and why is it so important.
Provides a listing of drugs. Type The group or category of approved drugs. The Orange Book is an FDA publication mandated under section 505j7A of the Federal Food Drug Cosmetic Act FDC Act which.
THE ORANGE BOOK thLecture Notes_Dr. Updated with Orange Book. Indias largest online marketplace for automobiles Droom has announced expansion of the scope of Orange Book Value popularly known as.
Approved Drug Products with Therapeutic Equivalence Evaluations. The Orange Book has long been a reliable resource for information about FDA-approved drugs. Definition It is the.
The orange book is published annually and the 2015 edition is 35th edition of orange. The orange book consist of five main sections. Book was published in October 1980 with orange cover and thus the name orange book.
Format is RX OTC DISCN. Read on to find out. The Orange book has been revised.
CDR Kendra Stewart RPh PharmD. The FDAs Orange Book is a significant book that is regarded as the authoritative reference for all matters pertaining to the administration of generic medication equivalents. On March 23 2020 FDA removed from the Orange Book the listings for biological products that have been.

Approved Drug Products With Therapeutic Equivalence Evaluations T H E O R A N G E B O O K Ppt Download

Medications That Require Special Handling Ppt Video Online Download
What Does Orange Book Mean Definition Of Orange Book Orange Book Stands For Approved Drug Products With Therapeutic Equivalence Evaluations By Acronymsandslang Com

Ppt The Fda Process For Approving Generic Drugs Powerpoint Presentation Id 239795

Generic The Unbranding Of Modern Medicine Jeremy A Greene

The Official Scrabble R Players Dictionary 7th Edition By Merriam Webster Paperback Target

Investigational New Drug Orange Book Understanding On 505 B 2 A

How Drug Life Cycle Management Patent Strategies May Impact Formulary Management

What Is Compendia S Impact On Product Decision Making Two Labs Pharma Services

What S In A Name Drug Nomenclature And Medicinal Chemistry Trends Using Inn Publications Journal Of Medicinal Chemistry

Drug Muggers Which Medications Are Robbing Your Body Of Essential Nutrients And Natural Ways To Restore Them Cohen Suzy 9781605294162 Amazon Com Books

Sickening How Big Pharma Broke American Health Care And How We Can Repair It Abramson John 9781328957818 Amazon Com Books
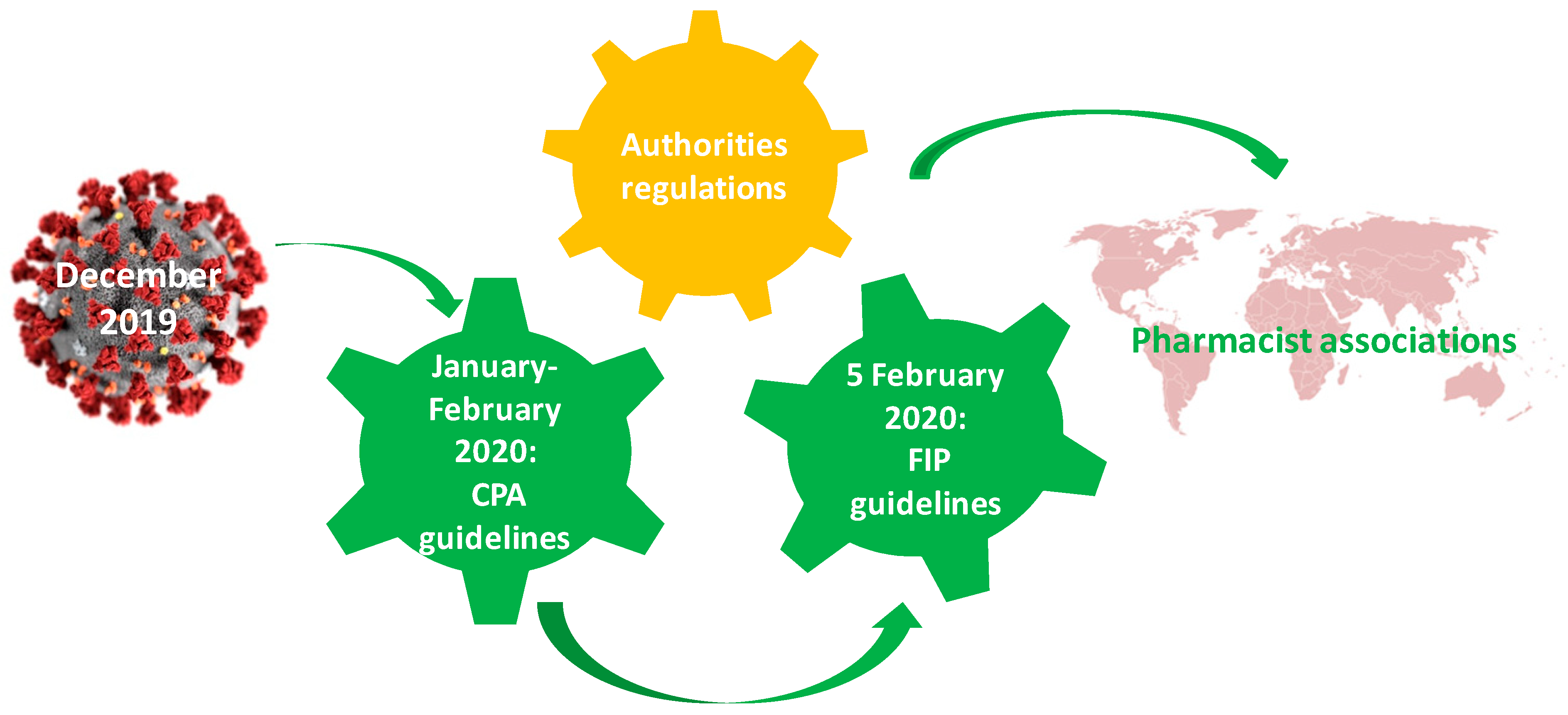
Ijerph Free Full Text The Particularities Of Pharmaceutical Care In Improving Public Health Service During The Covid 19 Pandemic Html
Association Between Us Pharmacopeia Usp Monograph Standards Generic Entry And Prescription Drug Costs Plos One

Orange Book And Its Applications Legal Advantage

Pha 6280 Medicare And Medicaid The University Of Florida College Of Pharmacy Department Of Pharmaceutical Outcomes Policy Course Coordinator Cody Ppt Download

